Integrated Endoscopy initiates worldwide pilot launch of single-use arthroscope
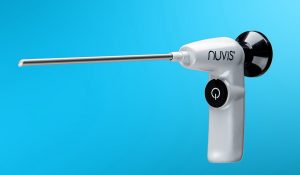
Integrated Endoscopy, a medical device company pioneering the development of high-definition, low-cost single-use endoscopes for the arthroscopic surgery market, today announced it has begun the international pilot launch of its NUVIS single-use arthroscope technology via placement in centers of medical excellence around the world.
News of the international roll out comes on the heels of Integrated Endoscopy’s clearance from the Food and Drug Administration (FDA) to market NUVIS in the United States.
NUVIS is a battery-operated arthroscope designed to provide high-definition visualization via a proprietary optical design, while directly addressing key safety and sterility issues associated with traditional, reusable arthroscopes. The single-use nature of the technology provides surgeons with an affordable way to eliminate bioburden and risk of infection and disease transfer due to lapses in instrument reprocessing; eliminates the need for the traditional light source and the associated heat; and can be used on existing video systems in the marketplace.
As part of this global strategy, Integrated Endoscopy has been working with a number of key hospitals and surgeons around the world, introducing the NUVIS technology. Among the early adopters globally are various centers located in the United Kingdom, France, India, Australia and a number of locations in the US.
Source: INTEGRATED ENDOSCOPY